
At what pressure will the volume of this sample be 11.02 L Assume constant temperature and ideal behavior.
757 mmhg to atm full#
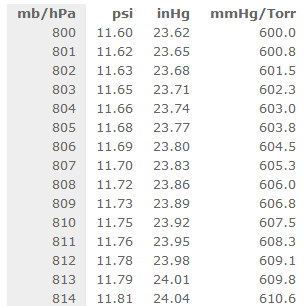
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types. atm 7569 mmHg Torr 1.880 am 722.9 KP atm 1322.2 mmHg Pa A certain mass of nitrogen gas occupies a volume of 8.64 L at a pressure of 6.04 atm. You can view more details on each measurement unit: mmHg or atm The SI derived unit for pressure is the pascal. We assume you are converting between millimeter of mercury 0 C and atmosphere standard. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. How many mmHg in 1 atm The answer is 759.9998769899. The unit is named after Blaise Pascal, the eminent French mathematician, physicist and philosopher.Ĭonversion calculator for all types of measurement units. The pascal (symbol Pa) is the SI unit of pressure.It is equivalent to one newton per square metre. The definition of a pascal is as follows: This small difference is negligible for most applications outside metrology.

The difference between one millimeter of mercury and one torr, as well as between one atmosphere (101.325 kPa) and 760 mmHg (101.3250144354 kPa), is less than one part in seven million (or less than 0.000015%). The relationship between the torr and the millimeter of mercury is: The decimal form of this fraction is approximately 133.322368421. the height of the liquid and the meniscus in millimeters are: 256. the vapor pressure of the water at 20 celsius is 17.5 mmHg. the pressure in mmHg exerted by the column of liquid in the burets were: 18.9, 21.8, 19.9. Therefore, 1 Torr is equal toġ01325/760 Pa. the temperature of the solution was 20 celsius. The millimeter of mercury by definition is 133.322387415 Pa (13.5951 g/cm3 × 9.80665 m/s2 × 1 mm), which is approximated with known accuracies of density of mercury and standard gravity. KPa to mm Hg, or enter any two units below: Enter two units to convert From: You can do the reverse unit conversion from


 0 kommentar(er)
0 kommentar(er)
